Tellurium, an essential component in photovoltaic solar cells, is about to be extracted as a byproduct of copper smelting at Rio Tinto’s Bingham (aka Kennecott) Mine, in Utah (Figure 1). The production of tellurium at Bingham, which is expected to begin in December 2021, will create a new North American supply chain for the critical element.

Introduction
In operation for 118 years, Rio Tinto’s Bingham Canyon Mine has produced more copper than any other mine in the world – ca. 20 million tons. Roughly 4 km wide and 1.2 km deep, Bingham is the largest manmade excavation and deepest open-pit operation in the world (Figure 2). Besides producing about 20% of the US supply of copper, the smelter at Bingham recovers gold, silver, platinum, palladium, selenium, and molybdenum. As a valuable step to critical mineral autonomy and energy security, the mine will begin to recover tellurium by the end of 2021.

What Is Tellurium?
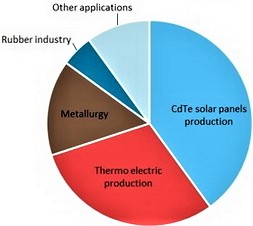
Tellurium is one of the least abundant elements in the Earth’s crust. It is present in most rocks at an average of about 3 parts per billion, which makes it rarer than gold and rare earth elements. Tellurium is a brittle, silvery-white element with properties in-between metals and nonmetals. It is used in semiconductors, electronics, ceramics, and catalysts (Figure 3). Tellurium is also utilized in alloys with steel and copper to improve ductility, and with lead to prevent corrosion. Tellurium’s primary application is to make thin films used in photovoltaic solar cells. These films are made of a cadmium-telluride (CdTe) alloy that efficiently absorb sunlight and convert it into electricity. Tellurium is also known for giving a garlicky odour to the breath. Common tellurium-bearing minerals include sylvanite (AuAgTe4), altaite (PbTe), calaverite (AuTe2), and petzite (Ag3AuTe2).
Due to its scarcity and wide distribution, mining exclusively for tellurium is not profitable. Since the critical element is often available as compounds with other commercially valuable metals (e.g., copper, gold), tellurium is typically recovered as a byproduct in the refining process of these other metals. Most tellurium comes from anode sludges produced in the electrolytic refining of copper, which average ca. 2 weight percent tellurium.
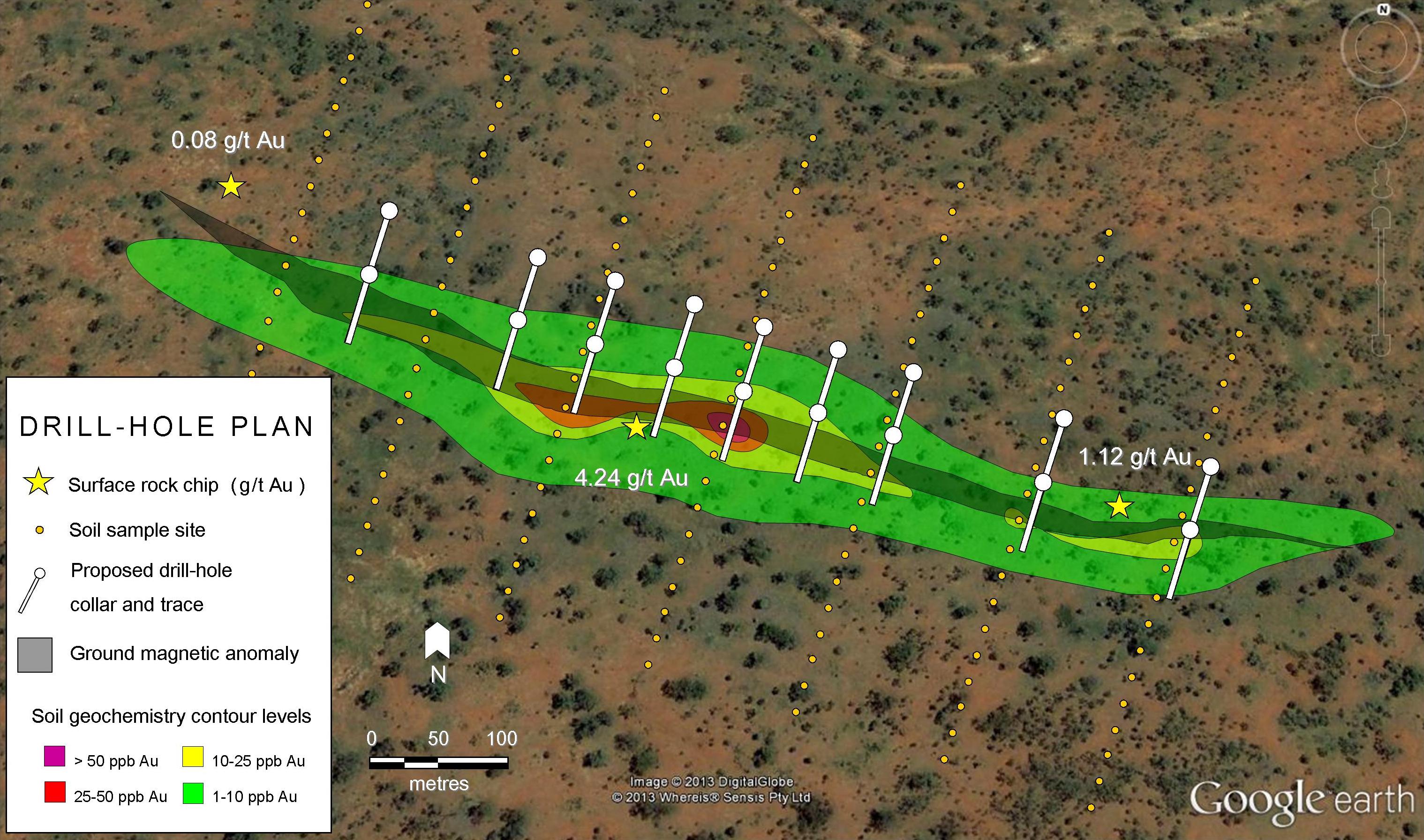
Overview of the Bingham Canyon (Kenecott) Mine
The Bingham Canyon is located in the central part of the Oquirrh Mountains, 30km southwest of Salt Lake City, Utah. The mine is owned by Rio Tinto (NYSE:RIO, ASX:RIO, LSE:RIO) and operated by its subsidiary Kennecott Utah Copper. As of February 2021, Bingham’s total mineral resources were projected as 243 Mt at 0.55% copper equivalent (CuEq) with a life of mine estimated to 2032.
History
The Bingham canyon was established in 1848 by the Bingham brothers, who were ranchers with no mining knowledge. The Bingham Canyon area became Utah’s first mining district in 1863 when soldiers stationed in Salt Lake City discovered lead ore in the canyon. Early works in the area indicated ore containing 2% copper. Large-scale exploitation of the canyon’s ore bodies using open-pit mining began by the end of the 19th century. In 1903, the Utah Copper Company was established to develop the mine with actual mining starting in 1906.
Regional Geology
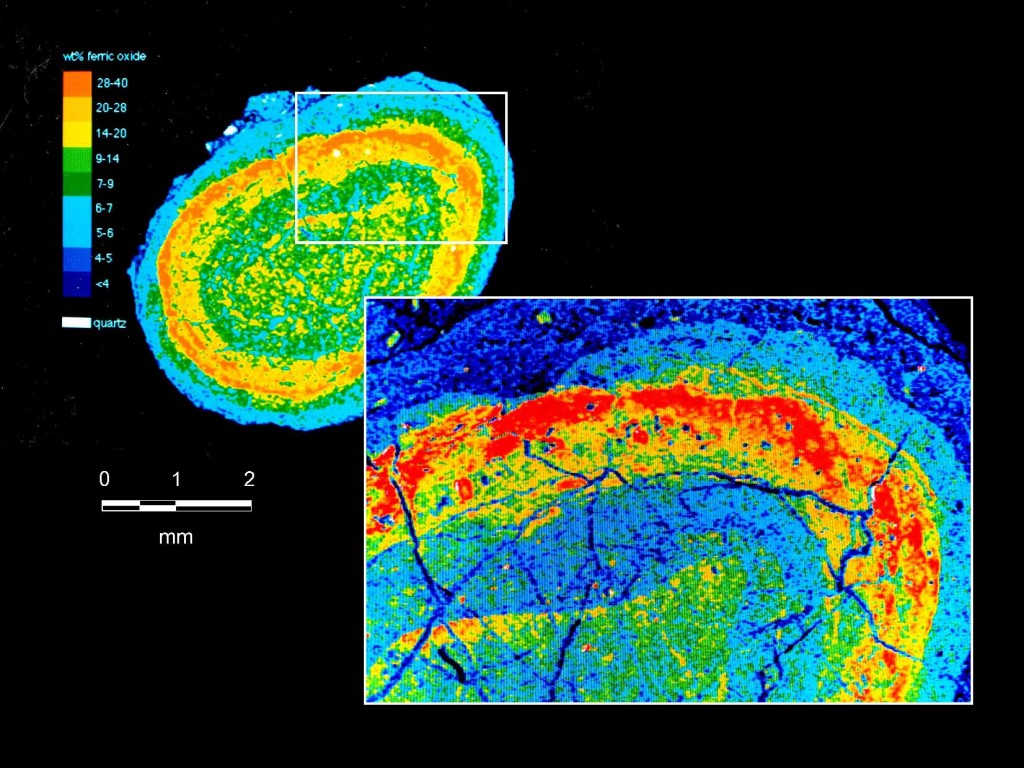
The geology of the Bingham Cu-Mo-Au deposit is dominated by igneous rocks of the multiphase Bingham stock (Figure 4). The intrusions and associated mineralization at Bingham are hosted by Pennsylvanian (323-298 million years) sedimentary rocks. The intrusive igneous rocks in the Bingham deposit range in age from 38.5 to 37.7 million years and comprise rock types including latite porphyry and monzonite (Figure 4).
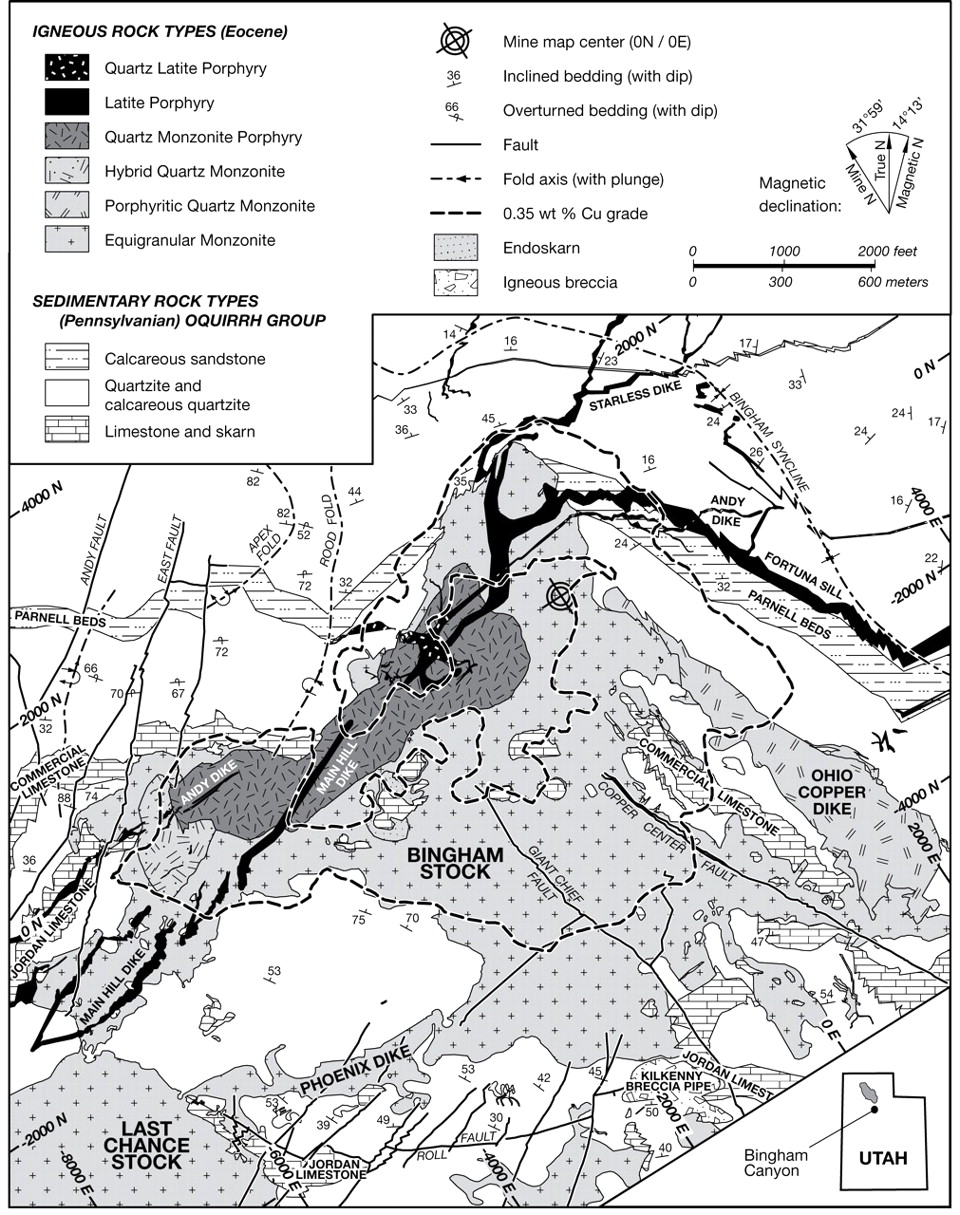
Deposit Type
Bingham is classified as a porphyry Cu-Mo-Au deposit. The massive deposit includes several ore types associated with porphyry Cu systems, such as Cu-Au-Mo porphyry, Cu-Au skarn, Zn-Pb-Ag-Au carbonate replacement, Pb-Zn-Cu-Ag-Au veins, and sediment-hosted Au (Figure 5). The mine is currently expanding to reach deeper mineral reserves along the southern flank of the open pit.
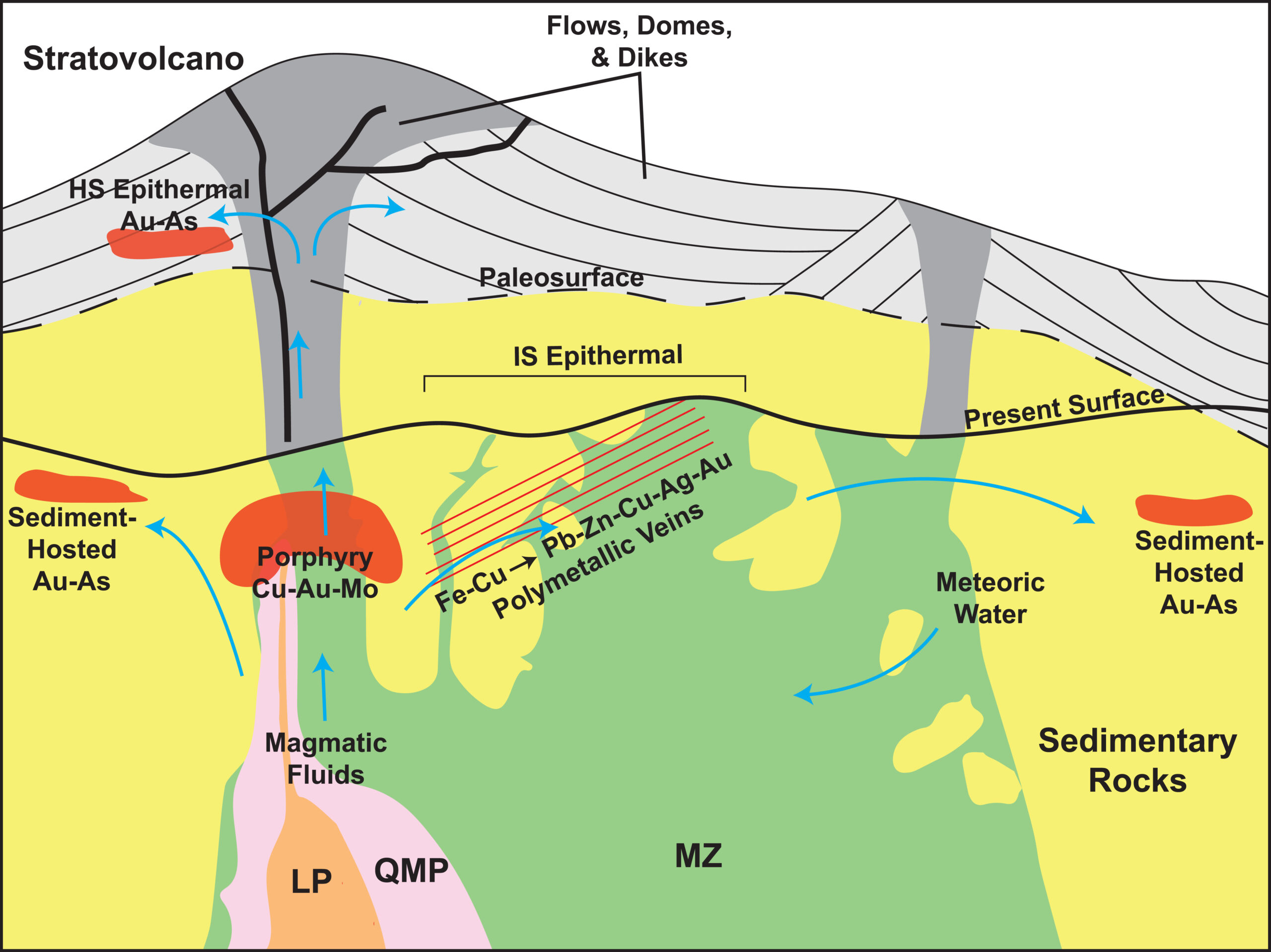
Mineralization
Mineralization and hydrothermal alteration at Bingham is consistent with traditional Cu-Mo porphyry deposits, with a Cu-barren, Mo-rich core – both of which are associated with potassic alteration. Based on cross-cutting vein relationships, the main Cu mineralization event is estimated to have occurred between the quartz monzonite porphyry and the latite porphyry intrusions. Mineralization is spatially and temporally zoned and alteration consists of a core of potassic alteration encircled by a large transitional zone to propylitic alteration with vein- and fracture-controlled sericitic and argillic alteration.
The Tellurium Recovery Process
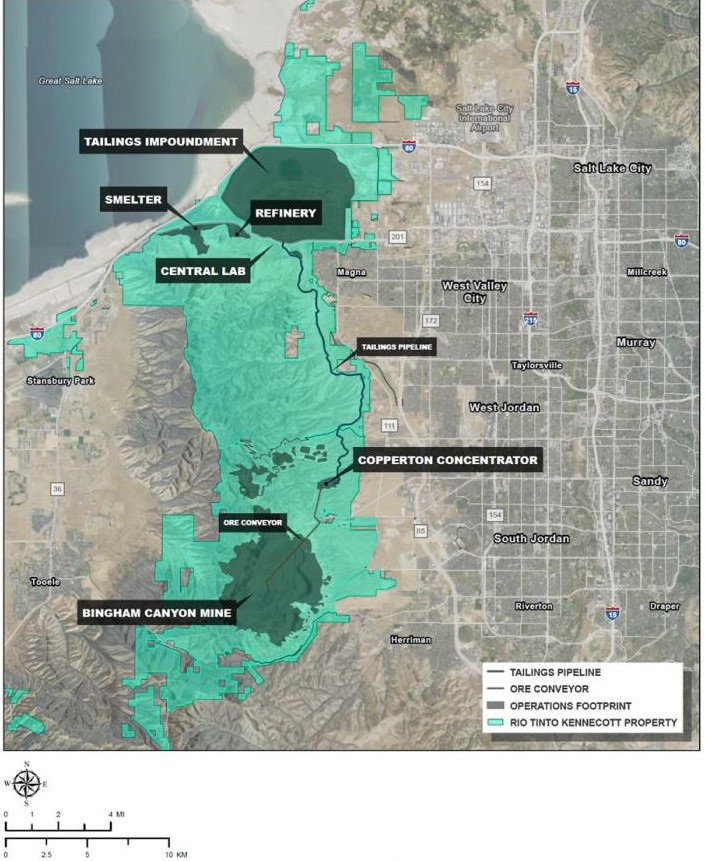
Kennecott Utah Copper operates the concentrator plant, smelter, and refinery at Bingham (Figure 6). Ore from the mine is transported on a conveyor belt to the Copperton Concentrator, where the material is ground and processed to separate waste from ore-bearing minerals. Copper concentrate is reduced into a slurry and sent to the smelter through a pipeline. At the smelter, the concentrate is processed in the furnace to produce anodes with 98.6% copper, which are sent to the refinery to form 99.99% copper plates. Tellurium is typically recovered from refined copper using an electrolytic process in which impurities present in high-grade copper ores are concentrated and removed from crude copper anodes.
Future Outlook/Investor Takeaways
Extremely rare, but an essential ingredient to photovoltaic solar panels, tellurium’s demand has been increasing substantially in the past few years. Despite still being the second most common technology used in photovoltaic panels, cadmium-telluride (CdTe) solar cells have gained space in the solar market as a cost-effective alternative to the traditional silicon-based photovoltaic cells. This is because they can be manufactured quicker and cheaper, and are less affected by dust, shade, and high temperatures. Due to the growing demand for renewable energy, tellurium is expected to reach “near-critical” supply levels by 2025 according to the US Department of Energy. Thus, more efficient tellurium extraction and new recovery processes may be critical to meet the growth in tellurium demand.
Further Reading
- USGS Tellurium Overview (pdf)
Companies Mentioned
- Rio Tinto (website)
Subscribe for Email Updates