Sediment-hosted Stratiform Copper (SSC) deposits have long been an important, but often overlooked, source of copper. Accounting for about 20% of global copper (Cu) production, SSC deposits are second only to porphyries in Cu and are also the fourth-largest source of silver (Ag) and the most important source of cobalt (Co).
SSC Deposit Characteristics
Whereas Cu porphyries are related to granitic intrusions and are located along convergent plate boundaries, SSCs are related to oxidized sandstone formations (redbeds) located in large sedimentary basins in the interiors of continents. In fact, SSCs have far more in common with unconformity-related uranium deposits than with Cu porphyries. SSCs have been found on every continent except Antarctica, but more than 80% of total resources contained in SSCs occur in the Central African Copper Belt in the Democratic Republic of Congo (DRC) and Zambia, and Kupferschiefer in Germany and Poland.
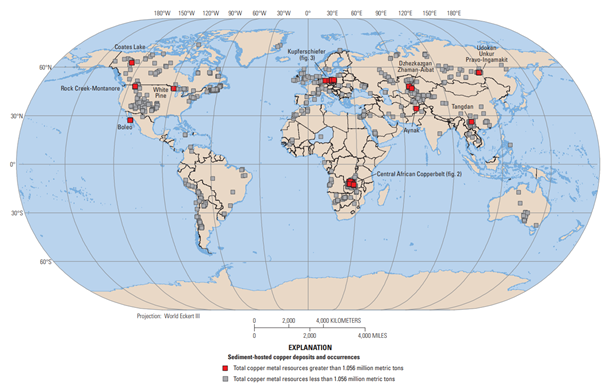
SSCs consist of Cu sulfide minerals disseminated or hosted in veins which are generally conformable with the sedimentary or metasedimentary host rocks.
They are divided into three sub-types based on host lithology:
- Reduced facies (Kupferschiefer) type deposits are hosted in shales or siltstones containing reduced carbon or sulfur minerals. They host sheet-like ore bodies of very large tonnage (sometimes 1 billion tonnes or more) and moderate grade (~1.4% Cu).
- In sandstone-type (Revett) deposits Cu minerals are hosted in sandstones containing hydrocarbons. Grades tend to be low (~1% Cu), with tabular or lens-shaped ore bodies.
- Redbed-type deposits are hosted in otherwise oxidized sandstones containing carbonized plant debris, and tend to be smaller (<10 Mt ore) but higher grade (~2% Cu) lens-shaped orebodies.
Ore bodies in all subtypes are strongly laterally zoned from pyrite (FeS2) to chalcopyrite (FeCuS2) to bornite (Cu5FeS4) to chalcocite (Cu2S) and finally to hematite (Fe2O3). Chalcocite and bornite are typically the main ore minerals; the higher Cu content of these minerals makes the ore easier and more profitable to process compared to typical chalcopyrite-rich porphyry ores. Approximately 27% of SSCs contain Ag with grades up to 200 g/t, and 14% contain Co with grades up to ~0.5%. These other metals are usually recovered as a byproduct of Cu mining. With very few exceptions, SSCs containing Ag don’t contain Co and vice versa.

Deposit Model
SSCs form in continental to shallow marine sedimentary basins containing thick sequences of redbeds. Such basins mainly form in hot, dry climates developed in areas of incipient rifting or post-mountain-building subsidence.
SSCs have a long history (mining of the Kupferschiefer deposits in Germany began in 1199), and mineralization was originally thought to have formed syngentically, with Cu minerals precipitating directly from river/ocean water at the time of sediment deposition. More detailed examination of the ore, however, led to the realization in the later part of the 20th century that the ore minerals formed diagenetically long after sediment deposition.
Cu minerals were precipitated from relatively cool (75-225 °C), oxidized, saline basinal brines which transported both the Cu and sulfur making up these minerals. These brines circulate through the porous redbeds of the host sedimentary basin, leaching Cu from sedimentary rocks, as well as any igneous or metamorphic rocks in contact with these sediments. Evaporites within the basin often contribute by acting as a source of sulfur as well as dissolved salts which increase the ability of basinal fluids to transport, and later precipitate, metals.
These metal-laden fluids eventually come into contact with more reduced rocks such as marine shales or carbonates, or the igneous or metamorphic rocks underlying the basin, triggering a chemical reaction where Cu-sulfide minerals are precipitated. This reaction occurs over some distance in both space and time, creating a distinctive zoning from hematite in the most oxidized areas to pyrite in the most reduced areas. The front of this reaction may shift over time, leading to the overprinting and replacement of reduced minerals by more oxidized ones.
The geometry of the ore body is mainly controlled by the geometry of the host sediments, with faults, folds, and other structural deformation exerting additional influence on mineralization by channeling fluid flow between oxidized and reduced rocks. Mineralization may be triggered by deformation of sedimentary basins during regional tectonic events, such as mountain-building. The large scale of the environments and processes involved in mineralization favors the formation of very large deposits and mineral districts, and the combination of relatively large tonnage, high grades, and predictable ore body geometry makes SSCs very attractive for large scale mining operations.

Other Metals In SSCs
Many elements such as lead, zinc, mercury, uranium, vanadium, and very rarely gold and Platinum Group Elements (PGEs) may be enriched in SSCs, but only Ag and Co are commonly produced. Cobalt, an essential component of modern batteries, may bring both rich rewards and potentially severe costs.
Cobalt is resistant to leaching and may become concentrated in weathered caps in the uppermost parts of SSCs, leading to valuable Co minerals being relatively accessible near the surface. With the DRC accounting for 70% of global Co, the country has been called the Saudi Arabia of electric vehicles. This, however, may be both an opportunity and a problem. The accessibility of these deposits, combined with the unstable political situation in the DRC, has led to the proliferation of small, primitive mines where unsafe work conditions, child labor, forced labor, organized crime, and environmental destruction often goes unchecked. Although most Co is now produced from large industrial mines, many of these are owned by state-run Chinese companies, leading to concerns of the global Co supply chain being monopolized for geopolitical gains, similar to the situation for rare earth elements.
The need for a more ethical and dependable Co source has been recognized for years, however no clear alternative is available. Deposits producing primarily Co are very rare, with most non-SSC Co produced as an extremely low-grade byproduct of nickel mining.
Notable Deposits And Districts
The most important deposits are located in the Central African Copperbelt of Zambia and the DRC. These reduced-facies and sandstone-hosted deposits have large tonnages and unusually high grades (~2.5%). Known resources in the belt are ~152 Mt Cu across more than 80 deposits, with the USGS estimating a further 168 Mt Cu remaining to be discovered, ~75% of which is in the DRC. These deposits are the world’s main source of Co, which adds significantly to the value of deposits.
The recent opening of the massive Khoemacau mine in the Kalahari Copperbelt in Botswana is a potentially significant development. The project, which entered production in June 2021, has total resources of 503 Mt at 1.4% Cu and 17 g/t Ag. The Kalahari Copperbelt appears to have much in common with the nearby Central African Copperbelt but has remained poorly explored until recently, and further large discoveries seem likely. Although substantial Co has yet to be discovered in the area, further exploration may help provide a solution for the world’s Co supply problem.
The Kupferschiefer deposits of western Poland and eastern Germany have produced much of Europe’s Cu for more than 800 years. The name comes from the German words for copper (Kupfer) and shale (schiefer). Since 1958 they have produced ~15 Mt Cu, with another 30 Mt awaiting development. The USGS estimates ~110 Mt Cu remains undiscovered, with the large majority in southwestern Poland. These reduced-facies deposits are hosted in thin, but very extensive, 255-million-year-old marine shales, and are unusual in that they produce a great diversity of byproduct metals including Ag, Co, Au, and PGEs. These mines have also produced many significant fossil discoveries.
Although not part of a large district like Kupferschiefer of the Central African Copperbelt, Michigan’s White Pine is the largest SSC in North America with indicated resources of 133.4 Mt at 1.07% Cu and 14.9 g/t Ag, and inferred resources of 97.2 Mt at 1.03% Cu and 8.7 g/t Ag. The original mine closed in 1997 after producing ~2 Mt at 1.17% Cu from reduced-facies ore. The property was 100% purchased Highland Copper Company in July 2021. Although the project is at a very early stage, it could produce ~2.2 billion lbs Cu and 27 Moz Ag over a 25-year mine life.
The Revett Formation in Montana and Idaho is also known also to host significant developed and undeveloped Cu-Ag resources in sandstone-style deposits. Similar deposits in the Colorado Plateau have also been mined for uranium and vanadium.
Conclusion
Although porphyries will remain the main source of Cu for the foreseeable future, the high grade and tonnage, and readily exploitable nature of SSCs makes these deposits increasingly attractive. With Cu prices at historic highs, the enormous undiscovered resources believed to exist and the rapidly increasing need for, and restricted supply of, Co only adds to the unique potential of these often-under-appreciated deposits.
List of Companies Mentioned
- Highland Copper Company (website)
Resources
- Highland Copper Company (2021): White Pine Project. (website)
- Niarchos, N. (2021): The Dark Side of Congo’s Cobalt Rush. The New Yorker. (news article)
- USGS (2014): Sediment-Hosted Stratabound Copper Assessment of the Neoproterozoic Roan Group, Central African Copperbelt, Katanga Basin, Democratic Republic of the Congo and Zambia (PDF)
- USGS (2015): New Kupferschiefer Copper Assessment Available (PDF)
- USGS (2015): Sediment-Hosted Stratabound Copper Deposit Model (PDF)
Subscribe for Email Updates