Introduction
Since the 2015 Paris Climate Agreement, there has been increasing pressure for the mining industry to reduce greenhouse gas emissions (GHG) and move towards carbon-neutral emissions. One of the goals of the Paris Agreement is to reduce GHG to keep global temperature increases under 2°C by 2100. Society must go beyond carbon neutrality and produce net negative carbon emissions to meet this goal. Recent research indicates that the mining industry could become a major player in achieving this end by capturing atmospheric CO2 in mine waste.
The vast majority (> 99 %) of rock excavated in mining ore is waste rock, also known as tailings. Such waste rock is typically pulverized and processed to remove valuable ore and then transported to permanent storage sites. At these sites, waste rock is disposed of in stockpiles or dams called tailing storage facilities (Figure 1). A common problem with mine waste is that it is inherently toxic to the environment. It can dramatically impact local water quality through the release of acidic or alkaline by-products, heavy metals, and fine dust.

As permanent storage sites, tailings storage facilities require maintenance in perpetuity, long after mines have closed. This occurs as an expense to mines, consuming 1.5 – 3.5 % of operating costs. In some cases, the costs of managing mine waste are high enough to render a project infeasible. On top of that, mining companies are pressured to find innovative ways to store their waste while meeting increasingly stringent environmental regulations and maintaining a social license to operate.
Emerging negative emissions technologies in the mining sector aim to reduce GHG and improve tailings storage in one fell swoop. Researchers are using a technique called mineral carbonation to actively mine CO2 from the air and store it permanently in the form of stable carbonated minerals. Mineral carbonation makes tailings storage more stable and environmentally friendly, as toxic heavy metals in tailings can be captured in carbonate minerals and stored permanently. This method is more robust than similar carbon storage technologies such as planting vegetation and injecting CO2 emissions underground as gas.
How Does Mineral Carbonation Work?
The basic reaction is straight forward: CO2 from the atmosphere reacts with magnesium- and calcium-bearing silicates and hydroxides to form carbonate minerals. This process already occurs naturally at the Earth’s surface as rocks undergo chemical weathering.
Unassisted, the natural formation of carbon-capturing minerals is a slow process, typically occurring over millions of years. But when ore is crushed for commodity extraction, the available surface area of the rock exposed to the air is increased by a factor of one million. In this setting, a reaction that typically takes one million years occurs in just one year!

It turns out that the accelerated extraction of CO2 from the atmosphere in mine tailings already occurs spontaneously in mine waste. As tailings sit exposed to air, they extract CO2, forming carbonate minerals as surface crusts and cement between mineral grains (Figure 2). This process is known as passive carbonation.
Research has also revealed this process is enhanced by the growth of biofilms (microorganisms) on the surfaces of tailings. Such bacteria extract CO2 from the air and secrete organic acids, further facilitating the breakdown of waste rock 100,000 times faster than non-biological processes. This discovery has led researchers to develop bioengineered approaches that harness the power of biofilms to accelerate mineral carbonation.
Some mines, such as BHP’s Mount Keith nickel mine in Australia (Figure 2), have offset their CO2 production by up to 11 % without even trying. With relatively minor modifications to how waste rock is stored and treated, a mine’s CO2 sequestration potential can be doubled easily.
Which Mines Are Best Suited for Mineral Carbonation?

Mines best suited to mineral carbonation are those that produce alkaline waste rock that is hosted by mafic or ultramafic rock. Mafic and ultramafic rocks are rich in magnesium and calcium-bearing minerals such as olivine, pyroxene, brucite, and talc. Alkaline waste has a high pH (low acidity) and will naturally react with CO2 to produce carbonate minerals.
Mines producing nickel, platinum group elements, diamonds, chromite, copper, aluminium, and talc have the greatest carbon storage potential. Collectively, these mining industries produce 419 million tons of tailings per year. If complete carbonation of those tailings were to occur, they could sequester 175 million tons of CO2 per year.
On the scale of individual operations, some mines have the potential to more than offset their own GHG emissions, thereby providing a sink for other emissions sources. Using the Mount Keith nickel mine as an example, complete carbonation of its 11 million tons of annually produced waste rock would store 4 million tons of CO2–10 times more than the GHG generated by the mine. The potential decrease in mines’ expenses by exploiting CO2 storage potential means that some mining operations could increase their economic feasibility and bring more projects online.
The mining industry has seen the benefits of mineral carbonation and companies are investing in this technology:
- In August 2020, Giga Metals (TSXV: GIGA) announced that it aims to make the Turnagain nickel project in the world’s first carbon-neutral mine using mineral carbonation.
- The De Beers Group (PLC [AAL.LN]) has been running pilot projects at its diamond mines in Canada and South Africa since 2016. Mining operations include Gahcho Kué, Victor, and Snap Lake in Canada and Voorspoed and Venetia in South Africa.
Future Potential
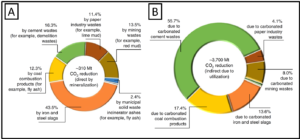
The sky’s the limit for the application of mineral carbonation in the mining industry. Carbon mineralization could lead not only to carbon-neutral operations but carbon neutrality for entire supply chains. Mines could also serve as CO2 sinks for other businesses that cannot afford to manage their own GHG emissions. Ultimately, successful adoption of carbon mineralization depends on future carbon pricing and GHG regulations. The market-based approach of cap-and-trade is widely considered the best option for mines to embrace carbon storage technologies. Operations that store carbon earn credits they can sell to those that exceed their emissions limits. This approach creates a larger pool of capital to support the growth of a carbon sequestration industry. Mineral carbonation shouldn’t be limited to the mining industry. Increasing research initiatives are applying this technology to other industries struggling to manage their waste, including cement, lumber, municipal waste, coal, and steel. Combined, they have the collective potential to reduce global CO2 emissions by 4010 million tons (Figure 3).
The successful reduction of GHG emissions on a global scale cannot be achieved without industrialization of carbon storage technologies. Mineral carbonation has the potential to turn mine waste into a significant financial asset for mining operations.
List of Companies Mentioned
Sources and Further Reading
- Bobicki ER, Liu Q, Xu Z, Zeng H (2012) Carbon capture and storage using alkaline industrial wastes. Progress in Energy and Combustion Science 38:302–320. (academic article)
- Mervine EM, Wilson SA, Power IM, et al (2018) Potential for offsetting diamond mine carbon emissions through mineral carbonation of processed kimberlite: an assessment of De Beers mine sites in South Africa and Canada. Miner Petrol 112:755–765. (academic article)
- Pan S-Y, Chen Y-H, Fan L-S, et al (2020) CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction. Nat Sustain 3:399–405. (academic article)
- Power I, McCutcheon J, Harrison A, et al (2014) Strategizing Carbon-Neutral Mines: A Case for Pilot Projects. Minerals 4:399–436. (academic article)
- Mineral Deposit Research Unit – CO2 Sequestration in Mine Tailings project (website)
Subscribe for Email Updates