Industrial mining is the foundation of modern society, everything that isn’t grown as a crop is ultimately derived from minerals or petroleum extracted from the Earth. Mining is also expensive and known to cause many environmental issues related mainly to toxic mine wastes and habitat destruction. In-situ leaching (ISL) methods, however, are increasingly allowing certain deposits to be mined without excavation, sidestepping some of the most destructive, and costliest, parts of mining.
What is ISL?
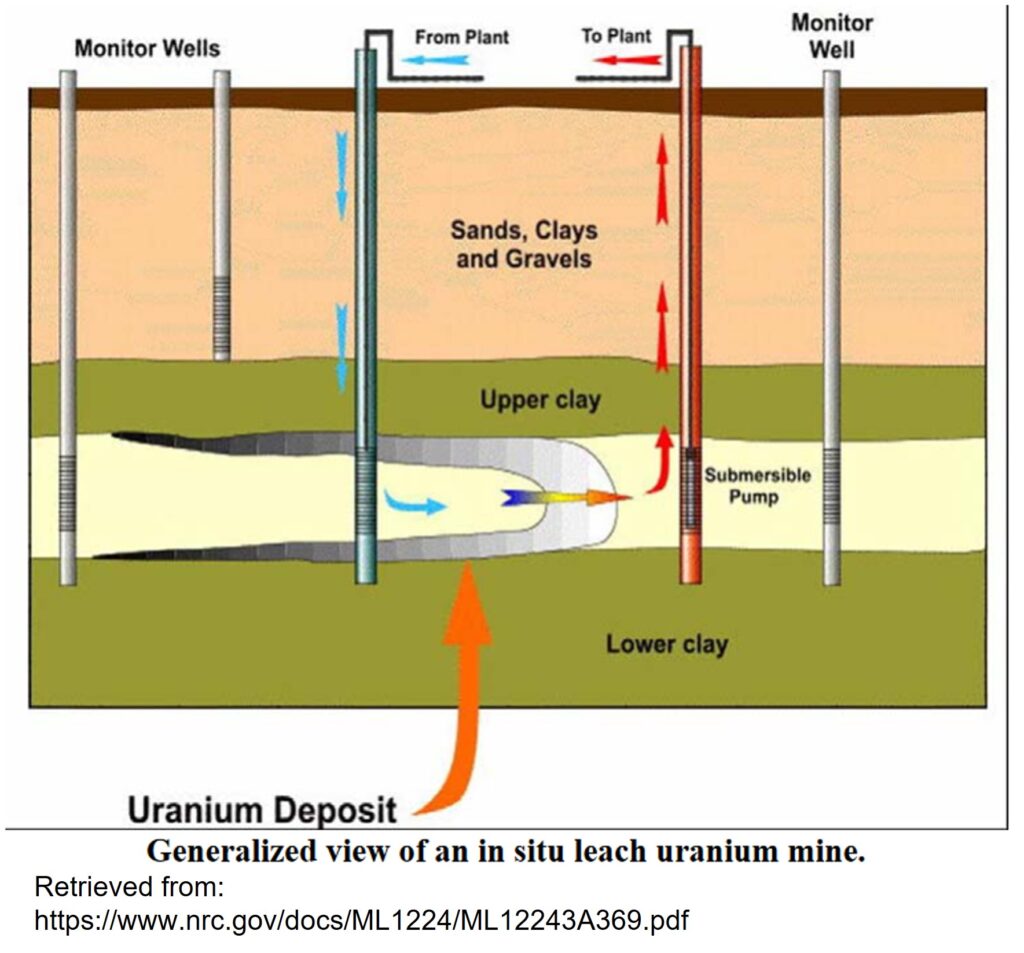
As the name implies, in-situ leaching involves the leaching and extraction of metals from underground ore bodies. The process is similar to heap leaching, where ore is extracted and piled up so leaching solutions can be circulated through it, except that ISL involves no excavation.
In general, ISL involves injecting a chemical solution, or lixiviant, into the ore body through an injection well; this solution circulates through the ore, dissolving the target minerals, and concentrating the metal of interest into a chemical brine. This brine is then pumped to the surface for processing via an extraction wells surrounding the injection site. This brine is then treated in a processing plant to extract the element of interest. Monitoring wells must also be installed around the mine to ensure groundwater is not being contaminated by the chemical brine.

There are several basic requirements for using ISL. First, the host rocks must be permeable enough for the solution to be pumped through it. This means that ISL in generally restricted to relatively shallow deposits hosted in unconsolidated sediments (ie. dirt), or sedimentary rocks. Second, the element of interest, and the mineral(s) it’s hosted in, must be soluble enough to be dissolved. In principle anything can be dissolved by the right chemical, but in practice only some minerals can be dissolved by readily available chemical solutions quickly enough to make ISL practical. Third, the flow of the solution must be controllable. This generally means the deposit has to be located below the water table and contained by impermeable (usually clay-rich) rocks. Finally, the deposit must not contain any minerals which will prematurely neutralize or otherwise react negatively with the solution.
Advantages and disadvantages
The main advantage of ISL is the fact that it skips the difficult and costly step of excavating large volumes of material. This means ISL mines require much less infrastructure and far fewer employees, keeping costs down and preventing workplace accidents. The lack of excavation also means no giant open pits or sprawling, potentially toxic tailings and waste rock piles; you could walk over an ISL mine without even realizing it. There are even major reductions in greenhouse gas emissions because ISL doesn’t require large fleets of diesel-powered equipment or energy-hungry transportation and grinding of ore. While ISL isn’t always an option, when it is it’s often the most cost-effective and environmentally friendly method: a rare win-win for miners and the environment.

The main disadvantage of ISL is how few deposits it can be applied to. Although work is underway to develop new techniques for more deposit types, there’s very little chance of ISL replacing conventional mining in most settings. Aluminum, for instance, is very difficult to dissolve, and will probably never be mined via ISL.
Since ISL takes place out of sight underground it can be difficult to understand and control the chemistry in detail. This means that when something goes wrong, low metal recovery for instance, it can be hard to figure out how to correct the problem.
In situ leaching isn’t completely free of environmental consequences either. The process creates large volumes of toxic used solution which must be disposed of. It also requires remediation once mining is complete. Remediation usually consists of extracting contaminated groundwater and injecting solutions designed to stabilize the newly disturbed chemistry of the deposit so that dissolved metals and other toxic byproducts don’t escape into the remaining groundwater. Remediation can take years, often as long or longer than the actual mining, and the process is still not fully understood.
What Deposits can be mined with ISL?
The most easily mined deposits are evaporites, such as halite (common salt) and sylvite (AKA potash, the source of potassium fertilizers), which can be easily dissolved by fresh water. In fact, the process can actually work too well, dissolution of too much material can leave large unsupported cavities that may collapse, creating sinkholes.
The metal most commonly mined via ISL is uranium (U). Uranium has the distinction of being highly soluble in oxygen-bearing water, and the addition of acids (sulfuric or less commonly nitric acid) or carbonate solutions allows most U minerals to be quickly dissolved. Not all U deposits are suited for ISL, pegmatite deposits, for instance, are too cohesive and impermeable to allow ISL solutions to circulate. Sandstone style deposits, on the other hand, which are hosted in highly permeable sandstones generally near the surface, are so well suited to ISL that there is little reason to even consider other methods. About half of global U production comes from ISL operations in Australia, the US, and, especially, Kazakhstan. Denison Mines has recently demonstrated highly successful tests of ISL on unconformity-related deposits in the Athabasca Basin, Canada, opening the exciting possibility of applying very low cost ISL methods to a deposit type which is extremely high grade but often challenging to mine conventionally.
Some copper (Cu) deposits can be mined via ISL, in fact ISL has been used to extract Cu in China for over 1000 years. Sulfuric or hydrochloric acid is used to dissolve certain Cu minerals such as malachite, azurite, tenorite, and chrysocolla. The most common Cu ore minerals such as chalcopyrite and bornite, however, are sulfides which can only be dissolved with the addition of oxidants such as oxygen. Even then, this process is slow, and ISL is used on relatively few Cu deposits.
In situ leaching is also used for some rare earth element (REE) deposits. The ion-adsorbed clay deposits of southern China and Myanmar are mined via (often primitive) ISL methods, and there is considerable interest in using ISL on other REE deposits hosted in permeable sediments and host rocks.
There have been attempts in mine gold, which is sometimes extracted via heap leaching, with ISL, but these have not been successful so far.
ISL in action
The Inkai mine is an excellent example of the potential of ISL. Inkai is a roll front (sandstone style) U deposit in Kazakhstan which is hosted in permeable sandstones at depth of about 250 m. Proven and probable reserves are 344 Mt at 0.04% U3O8. Mining commenced in 2009 and is expected to continue until 2045. It is 60% owned by Kazatomprom, which is majority owned by the Kazakh government, and 40% by Cameco.

Inkia uses a combination of oxidants and sulfuric acid to produce as much as 10.4 Mlbs of U3O8 per year, with recovery of 85%. Despite the very large size of the deposit, the extremely low grade would make Inkai very difficult mine economically via conventional methods. The total cost of ISL production, however, is remarkably low, less than 10$/lb, enabling the mine to operate successfully despite many years of low U prices and relatively low U recovery. Even more remarkably, the mine site is dominated not by pits and tailing ponds, but by grass and vegetation.
Summary
In-situ leaching allows for very low cost and environmentally benign mining of certain deposits. It is most often applied to certain types of U deposits, but can also be used for rare earths, Cu, and potentially others if the conditions are right. While the use of ISL is currently quite restricted, and the technique will never displace conventional mining in many areas, ISL is likely to be applied to a wider range of deposits in the future.
List of Companies mentioned
- Cameco https://www.cameco.com/businesses/uranium-operations/kazakhstan/inkai (website)
- Denison Mines https://denisonmines.com/ (website)
- Kazatomprom https://www.kazatomprom.kz/ (website)