Lithium (Li) is a metallic element that is most often found in granitic “pegmatites” (intrusive igneous rocks), brines (salty solutions), seawater and clays. In its pure form, it is soft light silver metal, but in nature it typically occurs as minor components of larger molecules or minerals.

Deposits and Extraction
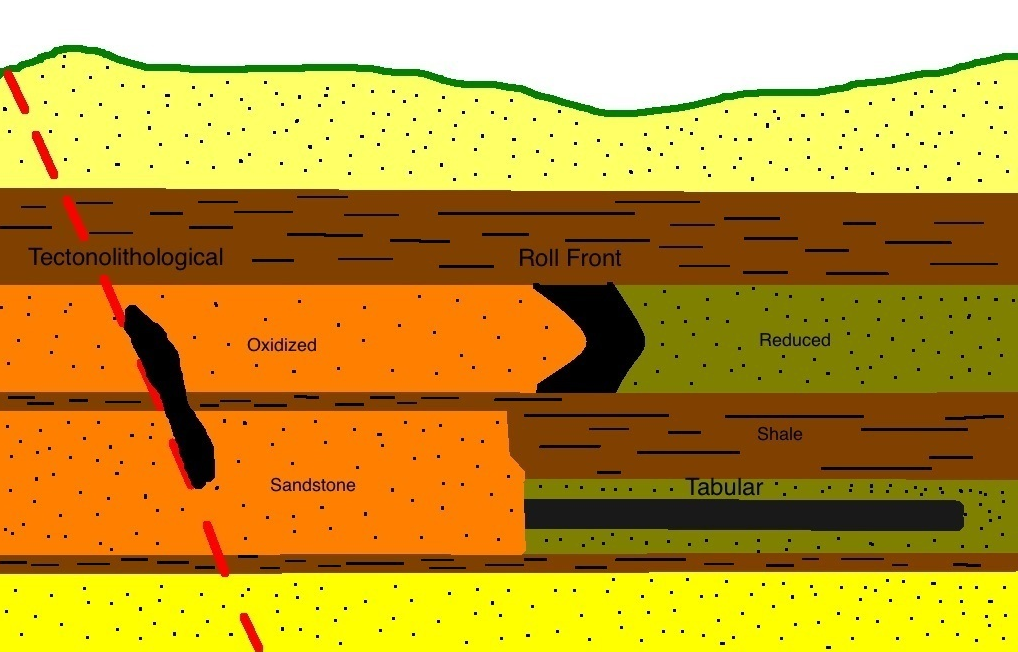
Lithium is mostly sourced from lithium-bearing brines (primarily as lithium carbonate) which are formed in saline lakes in desert environments. A prolonged period of natural enrichment and concentration is required to make these brines mineable.
Lithium minerals are also sourced from rocks know as pegmatites. Pegmatites are igneous rocks typically characterized by large crystals of its component minerals. Mineable deposits of exotic minerals and rare elements can occur in pegmatites including the tourmaline, emerald and aquamarine-bearing pegmatities of the Americas and a number of rare earth element deposits. The lithium in pegmatites occurs within silicate minerals such as spodumene and petalite, but also as amblygonite, lepidolite (a form of mica) and eucryptite. Lithium from these sources is more difficult to mine and generally more expensive compared to lithium sourced from brines.
Recently, seawater has also been considered as a source of lithium, but this has not been proven to be commercially viable. Hectorite clays have also been identified as a possible lithium source but production methods have not yet been proven.
Processing
Lithium is processed into lithium carbonate and lithium hydroxide. The production of lithium carbonate is primarily from brines, whereas lithium hydroxide is produced by hard rock mines. Lithium carbonate is much more cost effective to produce, but lithium hydroxide sells for a premium over lithium carbonate.
Markets
Lithium is used in batteries (about 30% of the market and growing), pharmaceuticals (used to treat gout and mental illness) and industrial markets (higher grade lithium is used in glass and ceramic manufacture). Both lithium hydroxide and lithium carbonate are used in the booming lithium battery market, with lithium carbonate currently the more important mineral. According to Benchmark Intelligence, Tesla’s new in-home ‘powerwall’ battery uses about 12kg of lithium per unit and Tesla has said that it’s new “Gigafactory” will require the world’s entire supply of lithium.

Contrary to popular belief, the largest producer of lithium is not China, but Australia (followed closely by Chile). Australian production is from pegmatite mine operations while large brine operations dominate Chilean production. Australia and China are the only countries that rely heavily on mining of lithium ores. According to the US Geological Survey 2015 Australia produced 13,000 tonnes of lithium in 2014, followed by Chile with 12,900 tonnes. These two countries make up about 72% of the world’s production and it is exported mostly to China, United States, Europe, Japan and South Korea; China remains a net importer.
Lithium prices are expected to grow with the industry attracting large amounts of attention and investment. Currently lithium carbonate is priced at $5,000-$6000 USD per tonne while lithium hydroxide sells from $7,000-$8,000 per tonne.
Further reading:
USGS summary on markets, uses, statistics (USGS pdf)
A preliminary deposit model for lithium brines (USGS pdf)
Tesla Gigafactory (Website)
Orocobre Chilean Brine Operation (Company Website)
Subscribe for Email Updates